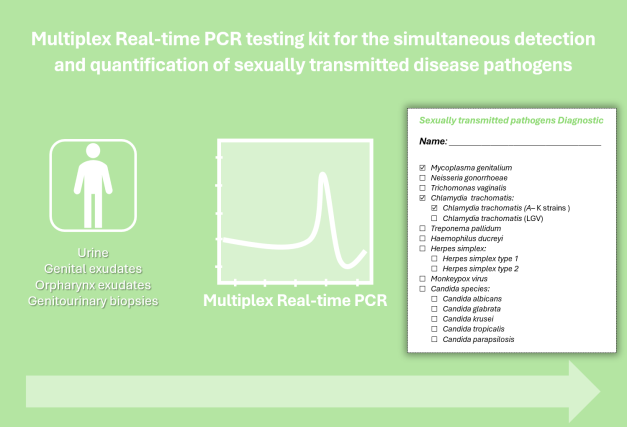
This invention consists of a set of primers and procedures for the simultaneous detection and quantification of sexually transmitted diseases (STD)-causing pathogens, as well as the Monkeypox virus and distinguishment of the two main Chlamydia trachomatis biovars. Additionally, it is easy to use, fast and can easily be incorporated into daily laboratory testing. Our method (multiplex real-time PCR) is highly sensitive and specific in the clinical detection of STD-causing pathogens, which will contribute to reducing STD-induced genital tract damage and improving public health worldwide. This testing kit can be used in both non-invasive (e.g. urine, genital and oropharynx exudates) and invasive samples (e.g. genitourinary biopsies).
Sexually transmitted diseases (STDs) are associated with an increased risk of genital cancer. Given the current challenges in their detection because they are often asymptomatic, it is crucial to develop fast, highly accurate and cost-effective diagnostic kits. This is essential to allow early detection and thus effective treatment and prevention of reproductive sequelae.
Highly sensitive, automated, fast and low-cost laboratory screening; Detection of STD-causing pathogens in both non-invasive and invasive test samples; Simultaneous detection and quantification of several STD-causing pathogens, as well as Monkeypox virus and distinction of the two main Chlamydia trachomatis biovars.
Clinical application - diagnostic tool for prevention and management of STDs






